Pharmaceutical contract manufacturing refers to the services performed by a contract manufacturer who produces goods or services for a pharmaceutical company. These services are outlined in a contract before the actual manufacturing takes place, ensuring profit for the contract manufacturer. Finding a pharmaceutical contract manufacturer is challenging because you will need to find one that can help keep costs low without curtailing the high-quality products that you will need in order to have a profitable, sustaining and successful business. Read More…
Since 1920, Hankscraft Inc. has been a leader in the contract manufacturing industry, producing dependable and high-quality products for all of our customers. We pride ourselves on our contributions to the industry and being industry experts. From plastics, to metals, to assembly we can manage your entire program.

Here at New Standard Corporation, we take great pride in our position as a leading provider of contract manufacturing solutions, offering a comprehensive range of products, services, and capabilities to address the diverse needs of our valued clients. With our unwavering commitment to excellence and extensive expertise in contract manufacturing, we stand ready to deliver innovative solutions that ...

PEKO Precision Products is an innovative twenty-first century contract manufacturer, with a vast set of capabilities and an expansive facility in Rochester, NY. Since PEKO’s founding in 1966 it has been providing crucial specialty manufacturing services to its customers, from sheet metal fabrication to piece part production, all the while investing in new technology and product development.

At Cretex Medical RMS, we specialize in delivering high-precision contract manufacturing solutions to the medical device industry. With decades of experience behind us, we’ve built a reputation for excellence in machining complex components and assemblies that meet the stringent demands of surgical and diagnostic applications. Our team works closely with OEMs to support every phase of the...

At AccraFab, some of our many capabilities include contract manufacturing, rapid prototyping, contract assembly and finishing. Our skilled machine operators can laser cut, punch, stamp, weld, form, assemble and package all the various types of metals we use: stainless steel, copper, aluminum and more.

More Pharmaceutical Contract Manufacturing
Pharmaceutical Manufacturing: Comprehensive Guide to Contract Services, Solutions, and Industry Applications
Pharmaceutical manufacturing plays a pivotal role in the life sciences sector, encompassing a broad range of contract services designed to streamline drug production, optimize quality, and ensure regulatory compliance. From producing active pharmaceutical ingredients (APIs) to packaging finished dosage forms, pharmaceutical contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs) provide essential support to biotechnology firms, pharmaceutical companies, and healthcare innovators.
With increasing competition and evolving regulatory requirements, many pharmaceutical companies are turning to specialized third-party manufacturers to support their operations. This trend is fueled by the need for operational efficiency, cost control, and access to advanced manufacturing technologies. Whether you're seeking tablet manufacturing, capsule filling, liquid formulation, sterile injectable production, or packaging and labeling solutions, understanding the landscape of pharmaceutical contract manufacturing is key to making informed sourcing decisions.
What Is Pharmaceutical Contract Manufacturing?
Pharmaceutical contract manufacturing involves outsourcing the production of pharmaceutical products to specialized third-party manufacturers. These CMOs and CDMOs offer a variety of services, including:
- Formulation development and process optimization
- API synthesis and scale-up
- Analytical testing and quality control
- Clinical trial material manufacturing
- Commercial-scale production of oral solids, liquids, semi-solids, and injectables
- Packaging, labeling, and serialization for compliance and supply chain traceability
Modern pharmaceutical contract manufacturing companies operate state-of-the-art facilities compliant with Good Manufacturing Practice (GMP) standards set by regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). By leveraging the expertise of CMOs and CDMOs, pharmaceutical firms can accelerate time-to-market, reduce capital expenditure, and focus on core competencies such as research and development (R&D).
Key Benefits of Pharmaceutical Contract Manufacturing
Outsourcing to pharmaceutical contract manufacturers offers several strategic advantages:
- Cost Reduction: Companies can avoid the high costs of facility maintenance, equipment investment, and workforce management. This is especially beneficial for small and mid-sized enterprises (SMEs) and emerging biotech firms.
- Access to Expertise: Contract manufacturers employ teams of highly skilled scientists, engineers, and regulatory specialists. They offer experience in process development, scale-up, and technology transfer, ensuring robust product quality and regulatory compliance.
- Scalability and Flexibility: CMOs can efficiently ramp up or scale down production volumes in response to market demand, clinical trial phases, or commercial launch requirements.
- Regulatory Compliance: Leading contract manufacturers adhere to global regulatory standards, offering comprehensive documentation and support for regulatory submissions such as New Drug Applications (NDAs) and Abbreviated New Drug Applications (ANDAs).
- Speed to Market: Contract manufacturing services enable faster product launches by reducing development timelines, minimizing bottlenecks, and leveraging established supply chain networks.
How Do Pharmaceutical Companies Choose the Right Contract Manufacturing Partner?
When evaluating pharmaceutical contract manufacturing organizations, decision-makers weigh several critical factors:
- Technical Capabilities: Does the CMO/CDMO have the technology, capacity, and expertise to handle your specific drug product, dosage form, or delivery system?
- Regulatory Track Record: Has the facility passed recent FDA or EMA inspections? Can they provide support for Investigational New Drug (IND), NDA, or ANDA filings?
- Quality Assurance: What quality management systems are in place, and how does the manufacturer ensure data integrity, batch consistency, and traceability?
- Project Management: Is there a dedicated project manager or team to keep your project on track and maintain clear communication?
- Geographic Location: Is proximity important for efficient logistics, supply chain management, or regulatory reasons?
- Cost Structure: Does the CMO offer transparent pricing, and do they have a history of delivering projects on time and within budget?
Are you comparing pharmaceutical contract manufacturing companies? Ask about:
- Batch release timelines and lead times
- Minimum order quantities and flexibility
- Technology platforms and innovation capabilities
- Support for serialization, anti-counterfeiting, and supply chain security
- Experience with your product class (e.g., small molecule, biologics, vaccines, or controlled substances)
Pharmaceutical Manufacturing Services: Core Offerings and Specializations
Pharmaceutical contract manufacturing covers a diverse array of services tailored to meet the needs of different drug products and market segments. Key offerings include:
1. Tablet and Capsule Manufacturing
Solid oral dose forms such as tablets and capsules remain the most common drug delivery systems. Contract manufacturers provide expertise in high-speed blending, granulation, compression, encapsulation, and coating. Specialized capabilities include modified-release formulations, multi-layer tablets, and combination products.
2. Liquid and Semi-Solid Formulation
Manufacturing of liquid syrups, suspensions, emulsions, creams, gels, and ointments requires controlled environments and precise process controls. CMOs employ advanced mixing, homogenization, and aseptic filling technologies to ensure product stability and safety.
3. Sterile Manufacturing and Injectable Production
Sterile injectables, including vials, ampoules, and prefilled syringes, demand stringent environmental controls and aseptic processing. Leading contract manufacturers operate cleanroom facilities with validated sterilization and containment systems, supporting both clinical and commercial supply.
4. Packaging, Labeling, and Serialization
Pharmaceutical packaging solutions are essential for product protection, patient safety, and regulatory compliance. Services include blister packaging, bottle filling, unit dose packaging, tamper-evident labeling, and integration with serialization systems for supply chain traceability.
5. Analytical and Quality Control Services
Comprehensive analytical testing is required to ensure drug identity, purity, potency, and stability. Contract labs provide method development, validation, release testing, stability studies, and microbiological analysis as part of integrated manufacturing solutions.
6. Product Development and Technology Transfer
From pre-formulation studies to process optimization and technology transfer, contract manufacturers support the entire product life cycle. Services include clinical trial material (CTM) manufacturing, scale-up, process validation, and regulatory documentation.
Use Cases and Applications: Who Uses Pharmaceutical Contract Manufacturing?
Pharmaceutical contract manufacturing is leveraged by a wide range of industry players, including:
- Large pharmaceutical companies seeking to optimize production capacity or focus on new drug development
- Biotechnology firms advancing novel therapies, such as biologics, cell and gene therapies, and biosimilars
- Generic drug manufacturers looking to produce cost-effective alternatives to branded medications
- Startups and virtual pharma companies that lack in-house manufacturing infrastructure
- Healthcare organizations requiring niche products or specialty compounding
Typical applications include clinical trial material manufacturing, commercial launch support, lifecycle management for mature products, and production of specialty pharmaceuticals such as orphan drugs and controlled substances.
Regulatory Compliance in Pharmaceutical Manufacturing
Regulatory compliance is non-negotiable in the pharmaceutical industry. Contract manufacturers must adhere to cGMP (current Good Manufacturing Practices) as outlined by the FDA, EMA, and other global authorities. Compliance involves rigorous documentation, validated processes, robust quality assurance, and regular audits. Failure to comply can result in regulatory action, product recalls, or loss of market access.
Considering regulatory compliance? Explore:
- The contract manufacturer's audit history and inspection outcomes
- Approaches to data integrity, electronic records, and batch traceability
- Support for regulatory submissions and post-approval changes
- Experience with global markets, including U.S., EU, and emerging regions
Pharmaceutical Contract Manufacturing vs. In-House Production: Decision Factors
How do you decide between outsourcing to a contract manufacturer and investing in internal manufacturing capabilities? Consider the following:
- Capital Requirements: Building or upgrading a GMP-compliant facility is capital-intensive. Outsourcing can eliminate the need for upfront investment.
- Resource Allocation: Focusing internal resources on R&D and commercialization may yield better returns than managing complex manufacturing operations.
- Speed and Flexibility: Contract manufacturers can rapidly adapt to changing project requirements, market dynamics, and regulatory updates.
- Risk Management: Partnering with an experienced CMO reduces operational, regulatory, and supply chain risks.
- Intellectual Property (IP): Ensure confidentiality agreements and robust IP protection when outsourcing sensitive projects.
Not sure whether to outsource pharmaceutical manufacturing? Ask:
- What is the total cost of ownership for in-house vs. contract manufacturing?
- Will outsourcing accelerate your project timeline or increase scalability?
- Does the CMO have experience with your product type and target market?
- How will you manage quality oversight and technology transfer?
Emerging Trends in Pharmaceutical Manufacturing
The pharmaceutical industry is rapidly evolving, driven by technological innovation, increasing complexity of drug products, and a globalized supply chain. Key trends shaping the future of contract manufacturing include:
- Continuous Manufacturing: Adoption of continuous processing technologies to improve efficiency, reduce costs, and enhance product quality.
- Personalized Medicine: Growing demand for custom therapies, including small-batch biologics, cell therapies, and precision medicine solutions.
- Advanced Analytics and Automation: Integration of digital technologies, including artificial intelligence (AI), machine learning, and real-time analytics for process optimization.
- Sustainability Initiatives: Increased focus on green chemistry, waste reduction, and energy-efficient manufacturing practices.
- Globalization and Supply Chain Resilience: Diversification of manufacturing partners, regionalization of supply chains, and strategies to mitigate disruptions.
Want to stay ahead in pharmaceutical manufacturing? Search for:
- Contract manufacturers with advanced technology platforms
- Partners experienced in personalized medicine and small-batch production
- CMOs/CDMOs committed to environmental sustainability and regulatory transparency
- Providers with integrated quality assurance, data management, and risk mitigation strategies
Closely Related: Medical Device Contract Manufacturing
Closely associated with pharmaceutical manufacturing is medical device contract manufacturing. Both sectors share an uncompromising focus on product integrity, safety, and compliance. Medical device contract manufacturers specialize in the design, assembly, and packaging of diagnostic devices, surgical instruments, wearable health technology, and implantable devices.
Medical device contract manufacturing involves:
- Precision engineering and prototyping
- ISO 13485-certified quality management systems
- Regulatory support for FDA 510(k) and CE Mark submissions
- Custom packaging, labeling, and sterilization services
- Post-market surveillance and lifecycle management
Just as with pharmaceuticals, the quality and reliability of medical devices can have life-altering consequences for patients. Selecting a reputable, experienced contract manufacturer is essential to ensure product safety, efficacy, and regulatory approval.
Pharmaceutical Contract Manufacturing Across Industries
Contract manufacturing services are not limited to the pharmaceutical and medical device sectors. They play a critical role in other highly regulated industries, including:
- Aerospace manufacturing – for precision components and systems
- Chemical manufacturing – for specialty chemicals and intermediates
- Electrical and electronic manufacturing – for circuit boards and assemblies
- Military and defense – for mission-critical equipment and supplies
Each industry demands rigorous quality assurance, traceability, and compliance with industry-specific standards. Pharmaceutical-grade contract manufacturing is often regarded as a benchmark for quality and process control in these adjacent sectors.
Frequently Asked Questions: Pharmaceutical Contract Manufacturing
What are the different types of pharmaceutical contract manufacturing services?
Services range from API synthesis and formulation development to analytical testing, packaging, and commercial-scale production. Some CMOs offer specialized capabilities in sterile manufacturing, high-potency compounds, or biologics.
How do I select the best CMO or CDMO for my pharmaceutical project?
Evaluate potential partners based on technical expertise, regulatory compliance, project management, facility capabilities, and experience with your product type. Request references, conduct site audits, and review their inspection history.
What is the difference between a CMO and a CDMO?
A CMO (Contract Manufacturing Organization) focuses on manufacturing services, while a CDMO (Contract Development and Manufacturing Organization) also offers product development, formulation, and analytical support in addition to manufacturing.
How do contract manufacturers ensure quality and regulatory compliance?
Leading CMOs and CDMOs implement robust quality management systems, validated processes, frequent internal audits, and comprehensive documentation to meet cGMP, FDA, EMA, and other global regulatory requirements.
Can contract manufacturers help with product development and clinical trials?
Yes. Many CDMOs provide end-to-end solutions, supporting clients from early-stage development through clinical supply and commercial launch.
Conclusion: Maximizing Value with Pharmaceutical Contract Manufacturing
Pharmaceutical contract manufacturing is a critical enabler of innovation, efficiency, and growth in the life sciences industry. By partnering with experienced CMOs and CDMOs, pharmaceutical companies gain access to world-class facilities, technical expertise, and regulatory support—while optimizing costs and accelerating product timelines.
Whether your organization is looking to outsource tablet manufacturing, sterile injectable production, or analytical testing, thorough due diligence and strategic partner selection are vital. Stay informed about industry trends, regulatory updates, and emerging technologies to ensure long-term success in pharmaceutical manufacturing.
Ready to connect with leading pharmaceutical contract manufacturing partners? Explore top pharmaceutical contract manufacturers to streamline your next project and bring your products to market with confidence.







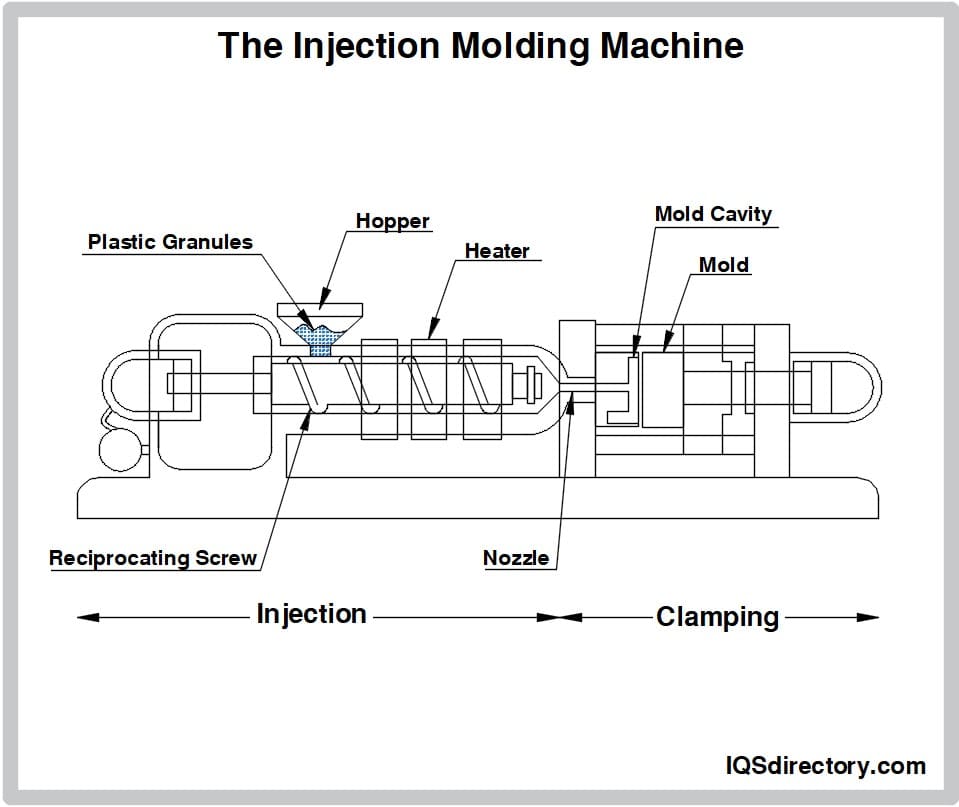
 Aluminum Extrusions
Aluminum Extrusions Broaching
Broaching CNC Machining
CNC Machining Expanded Metals
Expanded Metals Laser Cutting
Laser Cutting Metal Etching
Metal Etching Metal Fabrication
Metal Fabrication Metal Stampings
Metal Stampings Perforated Metals
Perforated Metals Screw Machine Products
Screw Machine Products Sheet Metal Fabrication
Sheet Metal Fabrication Steel Service Centers
Steel Service Centers Tube Fabrication
Tube Fabrication Water Jet Cutting
Water Jet Cutting Castings & Forgings
Castings & Forgings Bulk Material Handling
Bulk Material Handling Electrical & Electronic Components
Electrical & Electronic Components Flow Instrumentation
Flow Instrumentation Hardware
Hardware Material Handling Equipment
Material Handling Equipment Metal Cutting Services
Metal Cutting Services Metal Forming Services
Metal Forming Services Metal Suppliers
Metal Suppliers Motion Control Products
Motion Control Products Plant & Facility Equipment
Plant & Facility Equipment Plant & Facility Supplies
Plant & Facility Supplies Plastic Molding Processes
Plastic Molding Processes Pumps & Valves
Pumps & Valves Recycling Equipment
Recycling Equipment Rubber Products & Services
Rubber Products & Services