Contract manufacturers make products for an original equipment manufacturer, or OEM. The OEM’s name appears on the product when it is sold. Typically the medical device contract manufacture is not credited at all. Read More…
Hankscraft Inc. has been in the medical and pharmaceutical industries for decades. With our years of experience, we are able to provide our customers with a wide range of capabilities. This gives our customer the opportunity to fulfill all of their manufacturing needs through one company.

Here at New Standard Corporation, we take great pride in our position as a leading provider of contract manufacturing solutions, offering a comprehensive range of products, services, and capabilities to address the diverse needs of our valued clients. With our unwavering commitment to excellence and extensive expertise in contract manufacturing, we stand ready to deliver innovative solutions that ...

PEKO Precision Products is an innovative twenty-first century contract manufacturer, with a vast set of capabilities and an expansive facility in Rochester, NY. Since PEKO’s founding in 1966 it has been providing crucial specialty manufacturing services to its customers, from sheet metal fabrication to piece part production, all the while investing in new technology and product development.

At Cretex Medical RMS, we specialize in delivering high-precision contract manufacturing solutions to the medical device industry. With decades of experience behind us, we’ve built a reputation for excellence in machining complex components and assemblies that meet the stringent demands of surgical and diagnostic applications. Our team works closely with OEMs to support every phase of the...

At AccraFab, some of our many capabilities include contract manufacturing, rapid prototyping, contract assembly and finishing. Our skilled machine operators can laser cut, punch, stamp, weld, form, assemble and package all the various types of metals we use: stainless steel, copper, aluminum and more.

More Medical Device Contract Manufacturing
Contract Manufacturing for Medical Devices: Benefits, Challenges, and Strategic Value
In the highly regulated and competitive healthcare industry, medical device contract manufacturing has become an essential strategy for Original Equipment Manufacturers (OEMs) seeking to optimize operations, accelerate time-to-market, and maintain a focus on core competencies. By partnering with experienced contract manufacturers, OEMs can leverage specialized manufacturing expertise, advanced quality control systems, and scalable production capabilities, all while reducing costs and operational complexity.
Key Benefits of Contract Manufacturing for OEMs
The contract manufacturing transaction provides two primary benefits for the OEM. First, the OEM is assured that a competent and often certified manufacturer is producing its medical device, ensuring adherence to stringent regulatory compliance standards such as FDA, ISO 13485, and CE Mark requirements. Second, the OEM is freed from the responsibilities of manufacturing and is instead able to concentrate on the business side of things such as distribution, product development, and strategic marketing.
- Access to Specialized Expertise: Contract manufacturers bring extensive knowledge of medical device engineering, process validation, and supply chain management, enabling OEMs to develop innovative products while minimizing risk.
- Cost Efficiency: Outsourcing eliminates the need for large capital investments in facilities, equipment, and manufacturing staff, resulting in significant cost savings for both established companies and medical device startups.
- Scalability: Contract manufacturing organizations (CMOs) offer flexible production volumes, supporting OEMs through pilot runs, clinical trial batches, and full-scale commercialization.
- Quality Assurance: Professional CMOs implement robust quality management systems (QMS), ensuring every device meets or exceeds industry standards for safety and efficacy.
- Accelerated Time-to-Market: By leveraging existing manufacturing infrastructure and regulatory expertise, OEMs can launch new medical devices more quickly and efficiently.
Applications of Contract Manufacturing in the Medical Field
The contract manufacturers have the advantage of ensured profit, as the service is contracted. Many pharmaceutical and medical companies utilize contract manufacturing for a wide range of medical devices, from simple disposables to complex electromechanical systems. The medical field encompasses several distinct categories, each with unique manufacturing requirements and regulatory considerations.
Medical devices produced through contract manufacturing can be found in:
- Operating rooms (e.g., surgical instruments, diagnostic devices, anesthesia equipment)
- Labor and delivery units (e.g., fetal monitors, birthing beds, neonatal care devices)
- Nursing homes and long-term care facilities (e.g., mobility aids, wound care products)
- Intensive care units (e.g., ventilators, infusion pumps, patient monitoring systems)
- Home health care (e.g., blood glucose meters, portable oxygen concentrators, wearable sensors)
- Sleep labs (e.g., polysomnography equipment, CPAP machines)
- And more, including laboratories, imaging centers, and rehabilitation clinics
Medical devices can range from basic items like scalpels and suction tubes to stethoscopes, blood pressure monitors, intravenous (IV) sets, ultrasound equipment, implantable devices, and advanced diagnostic imaging technologies.
Industry-Specific Use Cases and Buyer Intent
Are you searching for medical device contract manufacturing services suitable for high-volume production, rapid prototyping, or small-batch clinical trials? Contract manufacturers offer tailored solutions for:
- Product Design and Development: Collaborative engineering support, rapid prototyping, and design for manufacturability (DFM)
- Assembly and Packaging: Cleanroom assembly, automated production lines, and sterile packaging for single-use and reusable devices
- Regulatory Support: Documentation, validation, and audit preparation for FDA, EU MDR, and global market entry
- Supply Chain Management: Sourcing of biocompatible materials, component procurement, and logistics optimization
- Aftermarket Services: Device refurbishment, field service support, and warranty management
Whether you are developing a Class I, II, or III medical device, choosing the right medical device CMO is critical for efficient commercialization and long-term product success.
Why Medical Companies Rely on Contract Manufacturing
Companies in the medical field benefit from contract manufacturing services particularly because their facilities' focus needs to be on serving the community with innovation and the most cost-effective devices, rather than taking up valuable space and resources with device production. By outsourcing manufacturing, healthcare technology companies can:
- Accelerate Innovation: Free up R&D and engineering teams to focus on new product development, clinical research, and breakthrough therapies
- Reduce Time-to-Market: Take advantage of established manufacturing processes, regulatory compliance expertise, and supply chain efficiencies
- Improve Product Quality: Rely on experienced manufacturers with rigorous process controls and continuous improvement systems
- Lower Operational Costs: Eliminate capital expenditures for plant equipment, facility maintenance, and skilled manufacturing labor
- Enhance Flexibility: Scale production up or down in response to market demand, seasonal fluctuations, or evolving healthcare needs
Also, the quality assurance and quality control achieved by hiring a professional medical device contract manufacturer is necessary and important when peoples' lives depend on the quality and integrity of a device. Contract manufacturers are often certified to ISO 13485 and other medical device manufacturing standards, ensuring robust documentation, traceability, and risk management in every step of the production process.
Potential Disadvantages and Important Considerations
Although contract manufacturing has several advantages, it has a few disadvantages as well. Understanding these potential risks is essential for making informed decisions when selecting a contract manufacturing partner:
- Loss of Intellectual Property (IP) Control: OEMs may face challenges in protecting proprietary designs, trade secrets, and confidential data, especially when partnering with offshore manufacturers. Clear contracts, non-disclosure agreements, and robust cybersecurity practices are critical safeguards.
- Communication Barriers: Language and cultural differences can create misunderstandings or delays. Establishing clear communication protocols and regular project updates helps mitigate these challenges.
- Reduced Oversight and Manufacturing Control: Relinquishing direct supervision over production can lead to concerns about process consistency, change management, and quality assurance. Selecting a reputable, transparent partner with a proven track record is key.
- Supply Chain Disruptions: Global events, raw material shortages, or logistics issues can impact delivery timelines. Risk assessments and contingency planning are essential components of a resilient outsourcing strategy.
The communication barriers, both lingual and cultural, are important to think about prior to signing a contract, especially when the contract manufacturer is foreign. Comprehensive due diligence, on-site audits, and transparent reporting structures can help alleviate many of these concerns. Ultimately, the advantages of contract manufacturing—including reduced costs, access to specialized expertise, and scalability—often outweigh the disadvantages when managed properly.
What Should You Look for When Choosing a Medical Device Contract Manufacturer?
Are you evaluating potential contract manufacturing partners? Consider these critical criteria to ensure a successful outsourcing relationship:
- Regulatory Compliance: Does the manufacturer hold relevant certifications (ISO 13485, FDA registration, EU MDR compliance) and demonstrate a history of passing audits?
- Technical Capabilities: Does the CMO offer advanced manufacturing technologies, such as precision machining, injection molding, microfabrication, or additive manufacturing (3D printing)?
- Quality Management: What quality assurance processes are in place? Are there robust systems for lot traceability, product testing, and risk mitigation?
- Experience and Track Record: Has the contract manufacturer produced similar devices or supported successful product launches in your target market?
- Scalability and Flexibility: Can the manufacturer accommodate changes in volume, design iterations, or regulatory requirements as your business grows?
- Intellectual Property Protection: What legal and physical safeguards are in place to protect your proprietary information and designs?
- Geographical Location: How does the manufacturer's location affect lead times, shipping costs, and responsiveness to market changes?
Asking these questions and performing thorough due diligence will help you select a partner that aligns with your business objectives and quality standards.
Industry Trends: The Future of Medical Device Contract Manufacturing
The global medical device contract manufacturing market is projected to grow steadily, driven by factors such as increasing demand for minimally invasive devices, rising healthcare expenditures, and the proliferation of connected health technologies (IoT medical devices). Contract manufacturers are investing in advanced automation, digital manufacturing, and data analytics to deliver greater efficiency, traceability, and product customization.
- Adoption of Smart Manufacturing: Integration of Industry 4.0 technologies—such as robotics, real-time monitoring, and predictive maintenance—enhances quality and reduces time-to-market.
- Focus on Sustainability: Eco-friendly materials, energy-efficient processes, and waste reduction initiatives are increasingly important selection criteria for OEMs.
- Emergence of Digital Health Devices: Wearable sensors, remote monitoring systems, and telemedicine platforms require specialized manufacturing expertise and regulatory knowledge.
- Expansion of Value-Added Services: Leading contract manufacturers now offer end-to-end solutions, including product design, supply chain optimization, and aftermarket support.
Are you keeping pace with these industry trends? Consider how your choice of contract manufacturing partner can support your strategic growth, regulatory compliance, and product innovation goals.
Frequently Asked Questions About Medical Device Contract Manufacturing
- What is the difference between a contract manufacturer and an OEM?
An OEM (Original Equipment Manufacturer) owns the product design and brand, while a contract manufacturer (CMO) is contracted to produce the product according to the OEM's specifications. Many medical device companies operate as OEMs, outsourcing production to specialized CMOs. - How do I ensure regulatory compliance when outsourcing device manufacturing?
Select a contract manufacturer with proven compliance to global standards (FDA, ISO 13485, EU MDR) and a strong history of successful audits. Close collaboration on documentation and validation is essential. - Can contract manufacturers assist with product development and prototyping?
Yes, many CMOs offer engineering support, rapid prototyping, and design for manufacturability (DFM) services to help bring your medical device concept to market efficiently. - What are typical contract manufacturing costs?
Costs vary based on device complexity, materials, production volume, and required certifications. Obtaining detailed quotes and cost breakdowns from multiple CMOs is recommended. - How can I protect my intellectual property when working with a CMO?
Use comprehensive contracts, NDAs, and select reputable partners with established IP protection policies and secure IT systems.
Ready to Explore Medical Device Contract Manufacturing?
If you're considering outsourcing your medical device production, start by defining your project requirements and identifying potential partners with the right capabilities and certifications. Compare quotes, request case studies, and assess each manufacturer's track record in similar device categories. Strong collaboration and clear communication are key to successful partnerships in medical device contract manufacturing.
Take the next step:
- Find qualified medical device contract manufacturers
- Request a quote for your next device production run
- Download our guide to evaluating contract manufacturing partners
- Explore case studies of successful OEM-CMO collaborations
By leveraging the strengths of experienced contract manufacturers, medical device OEMs can achieve faster time-to-market, lower production costs, and uncompromising product quality—supporting better outcomes for patients and healthcare providers worldwide.
For more information about contract manufacturing services, industry trends, and best practices, contact our team or explore our resources on medical device manufacturing, regulatory compliance, and supply chain optimization.







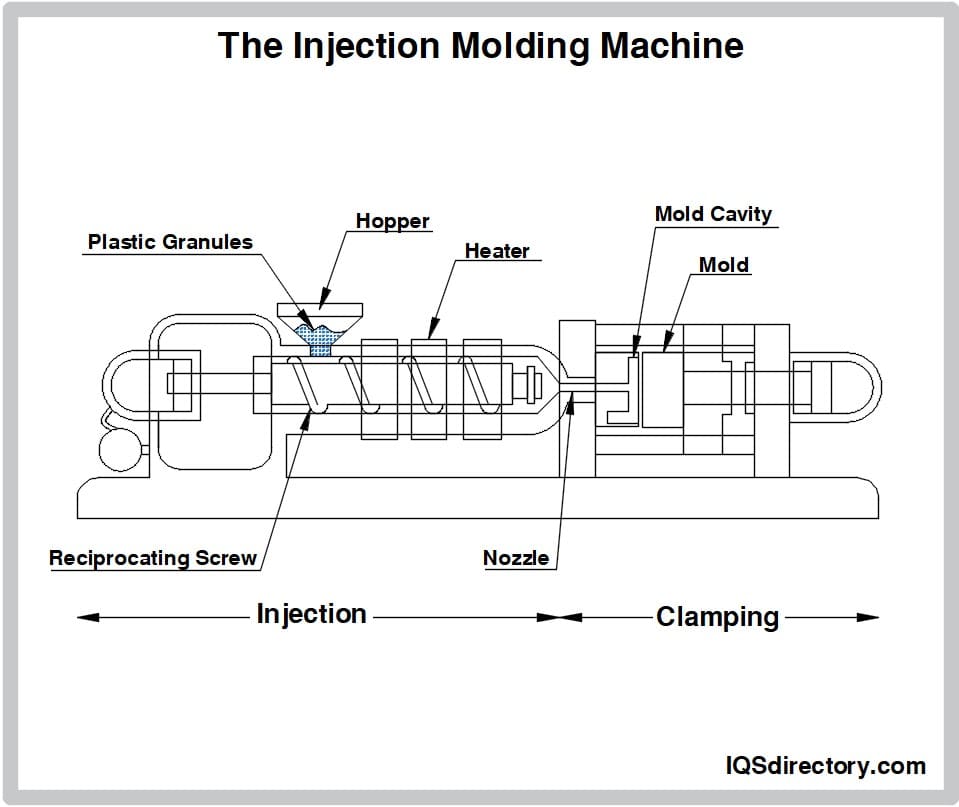
 Aluminum Extrusions
Aluminum Extrusions Broaching
Broaching CNC Machining
CNC Machining Expanded Metals
Expanded Metals Laser Cutting
Laser Cutting Metal Etching
Metal Etching Metal Fabrication
Metal Fabrication Metal Stampings
Metal Stampings Perforated Metals
Perforated Metals Screw Machine Products
Screw Machine Products Sheet Metal Fabrication
Sheet Metal Fabrication Steel Service Centers
Steel Service Centers Tube Fabrication
Tube Fabrication Water Jet Cutting
Water Jet Cutting Castings & Forgings
Castings & Forgings Bulk Material Handling
Bulk Material Handling Electrical & Electronic Components
Electrical & Electronic Components Flow Instrumentation
Flow Instrumentation Hardware
Hardware Material Handling Equipment
Material Handling Equipment Metal Cutting Services
Metal Cutting Services Metal Forming Services
Metal Forming Services Metal Suppliers
Metal Suppliers Motion Control Products
Motion Control Products Plant & Facility Equipment
Plant & Facility Equipment Plant & Facility Supplies
Plant & Facility Supplies Plastic Molding Processes
Plastic Molding Processes Pumps & Valves
Pumps & Valves Recycling Equipment
Recycling Equipment Rubber Products & Services
Rubber Products & Services